Benefits
Specifications
How-to
Contact Us
Learn More
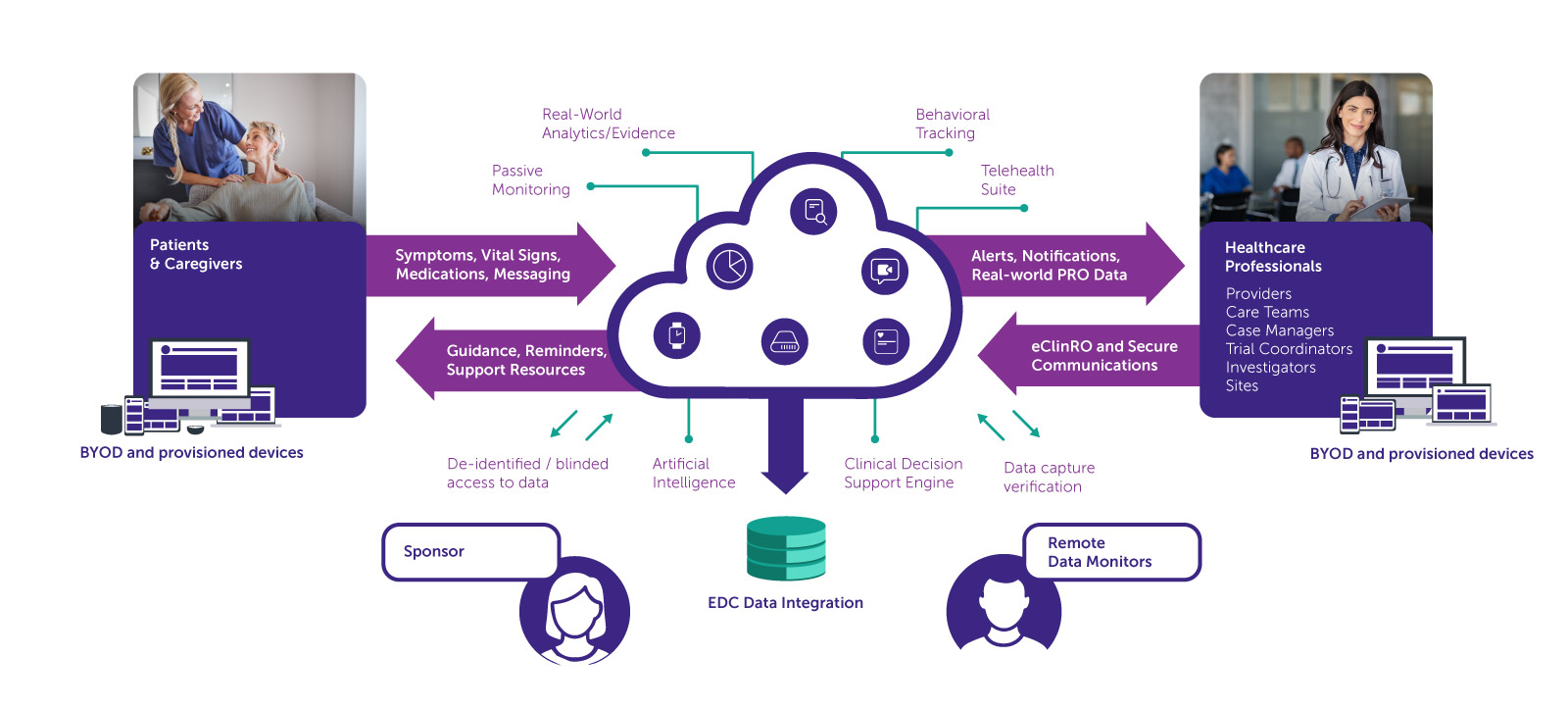
One Connected Ecosystem to Power Every Phase of Healthcare Research
Evidence Capture System ™ [ECS™] is a next-generation virtual research ecosystem designed to streamline research from start to finish. It blends fully remote study capabilities with phygital (physical + digital) flexibility, creating a seamless environment where researchers, participants, and sponsors collaborate in real time.
Faster Science, Stronger Evidence
Through end-to-end digitalization of all study touchpoints — from direct-to-participant onboarding and eConsent to real-time data acquisition and centralized monitoring — ECS™ optimizes study execution while maintaining strict adherence to GXP, data integrity principles, and international regulatory frameworks.
Architected for full scalability, ECS™ supports a wide spectrum of protocol designs:
Multicentric
Multi-country interventional trials
Observational research
Real World Evidence
High-frequency scientific data collection initiatives
Built for Flexibility. Designed for Impact.
ECS™ combines three seamlessly integrated modules that empower participants, streamline data collection, and support AI-enhanced research coordination. Whether your study is decentralized, hybrid, or site-based, ECS™ adapts to fit your protocol and goals.
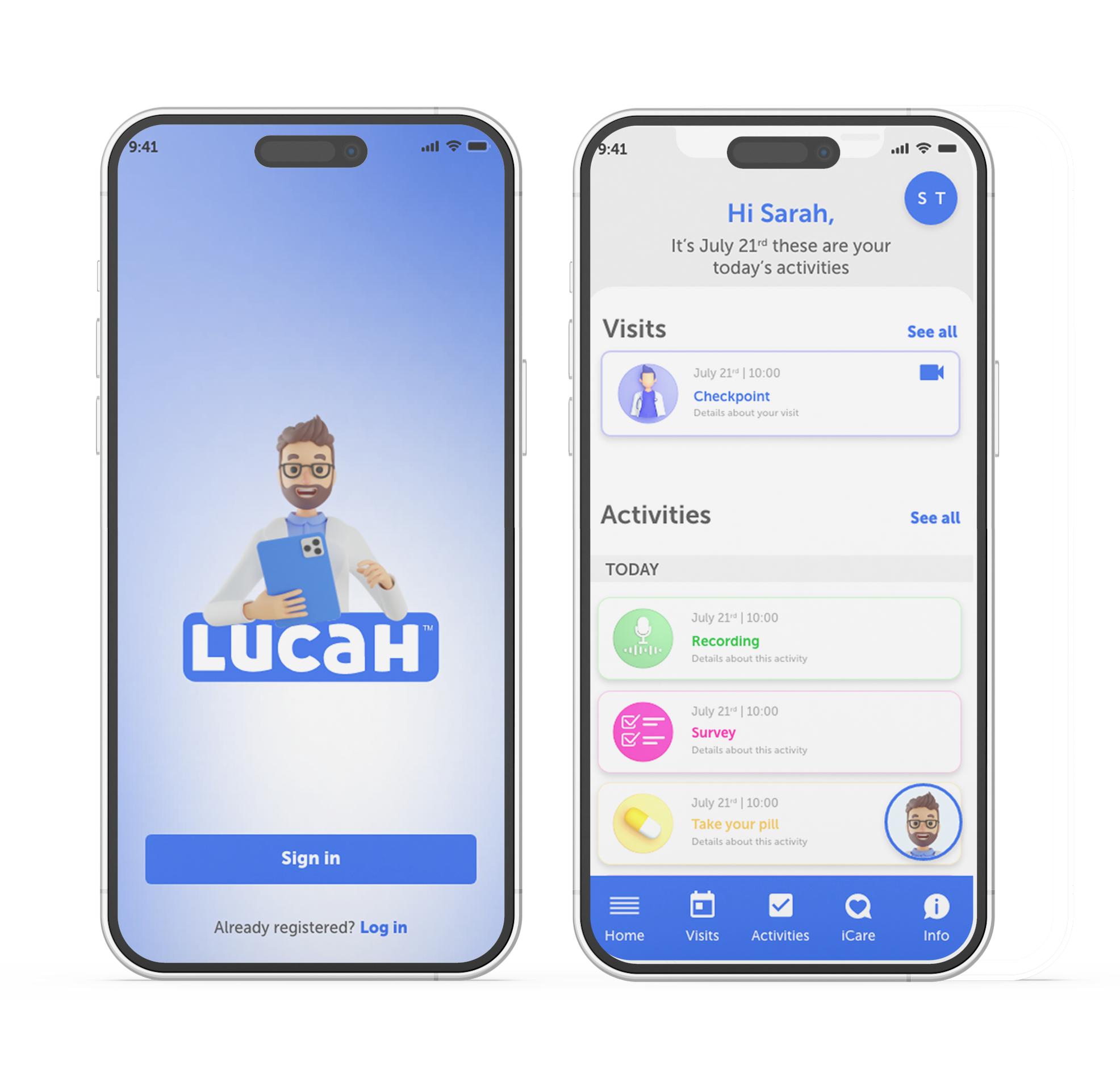
Lucah™ app
ECS™ maintains continuous participant interaction through digital reminders, mobile notifications, and live support—reducing dropout rates and improving data reliability across long-term studies.
On-Boarding
Managing people data means making them fully comfortable. The virtual home-based functionalities for D-T-P engagement process can allow Lucah™ App to manage the onboarding and eConsent phase remotely.
Study Journey
Creating a comfortable Journey when the Study is oftentimes a burden in our fast-paced society and busy lifestyle. The multichannel collection of Participant’s health data at the touch of a button: eQuestionnaires, audio/image/video capture and wearable device integration.
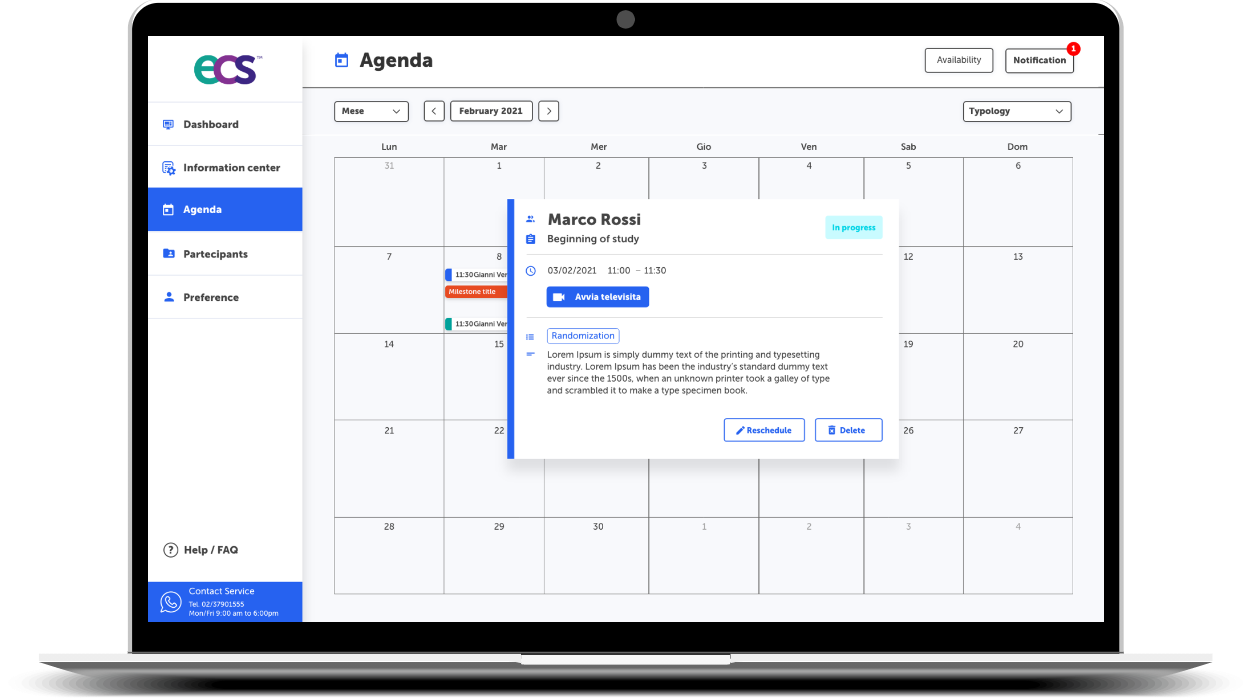
Study agenda planning
What if the Participant misses the IP or an assigned Study task? Forgetfulness is a Human feature, not a bug; so here is how Lucah™ can help. Schedule Study Events and Tasks and set reminders/notifications to ensure the Participant’s compliance and retention are maintained throughout the Study Journey.
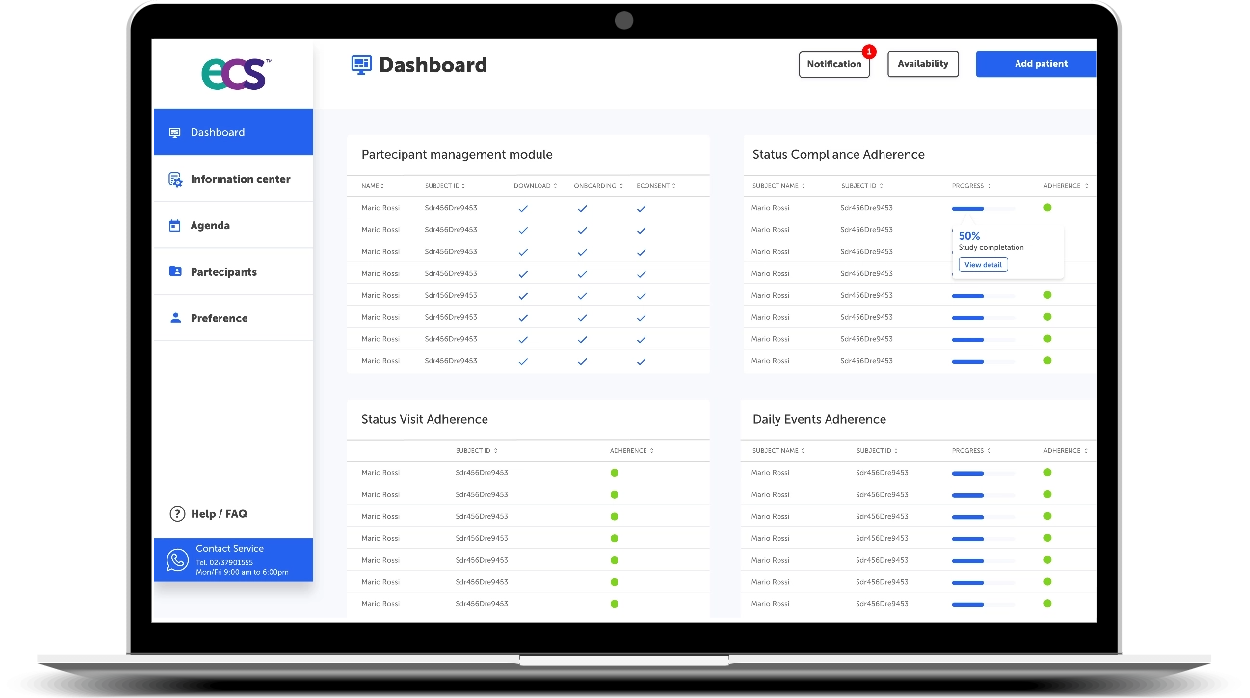
Data Capture Module
Looking to engage with and manage remotely Participants through Digital Health while having access to their health data in real-time?
Looking to manage eCRF, eCOA/TeleCOA, eClinRo, eObsRo, ePerfO, ePRO, Health Surveys, eDiary, PRO/ePRO, PROM/ePROM?
The Data Capture Module can handle and roll out in an innovative and certified way all these tools with a superior user experience.
Telehealth
Integrating telehealth with other virtual health solutions and in-person/hybrid/virtual care models, to improve patient/participant experience, convenience, outcomes, and affordability.
eCRF
Flexibility and rapid set-up time of eCRFs that facilitate the collection of accurate observations and reliable findings, and support transparency by providing real-time access to data.
Remote management
The remote management better engages patients/participants in experiencing the study journey, and gives the doctor a clearer window into their progress.
Interactive supply chain tool
A comprehensive flow of D-T-P (Direct to Participant) and D-F-P (Direct from Participant) processes for IP delivery and adherence eTracking, thanks to the direct connection with certified courier for sample delivery & recollection to and from home.
Virtual Intelligent Study Assistant Modules (ViSA)
ECS™ maintains continuous participant interaction through digital reminders, mobile notifications, and live support—reducing dropout rates and improving data reliability across long-term studies.
Workflow Automation
From visit scheduling and automated reminders to data validation and quality checks, ECS™ reduces manual tasks across the study lifecycle. Your team can focus on science, not spreadsheets.
Seamless User Experience
Participants use the Lucah™ mobile app to report data, receive reminders, and attend tele-visits—all from their own device. Researchers and sponsors benefit from tailored dashboards and role-based interfaces, ensuring clarity, speed, and usability at every level.
Purpose-Built Tools for Every Stakeholder
ECS™ is not one-size-fits-all. Investigators, monitors, and sponsors each get customized access to data and study tools, while participants engage through a secure, intuitive digital environment—boosting retention and protocol adherence.
AI-Powered Virtual Study Assistant
24/7 Support
ViSA provides continuous assistance to both participants and researchers, ensuring questions are answered at any time.
Automated Scheduling
Coordinates appointments, sends reminders, and manages follow-ups to keep studies on track.
Intelligent Chatbot
Answers protocol questions, delivers health surveys, and provides personalized guidance throughout the study.
Reduced Admin Load
Handles repetitive tasks automatically, allowing research staff to focus on higher-value activities.
Transform Your Next Trial with ECS™ – Connect with us
Participant-facing mobile application for ePRO, eDiary, and telehealth visits
Get in Touch
Contact our team to discuss your specific needs
Data Capture & ViSA
Experience the platform in action
Launch Your Study
Transform your research with next-gen technology

What is ECSTM
Why ECSTM
Solutions
Therapeutic areas
Case studies
Compliance
Privacy Policy
Cookie Policy
Copyright @2025 Evidilya srl. All rights reserved.

Benefits
Specifications
How-to
Contact Us
Learn More

One Connected Ecosystem to Power Every Phase of Healthcare Research
Evidence Capture System ™ [ECS™] is a next-generation virtual research ecosystem designed to streamline research from start to finish. It blends fully remote study capabilities with phygital (physical + digital) flexibility, creating a seamless environment where researchers, participants, and sponsors collaborate in real time.
Faster Science, Stronger Evidence
Through end-to-end digitalization of all study touchpoints — from direct-to-participant onboarding and eConsent to real-time data acquisition and centralized monitoring — ECS™ optimizes study execution while maintaining strict adherence to GXP, data integrity principles, and international regulatory frameworks.
Architected for full scalability, ECS™ supports a wide spectrum of protocol designs:
Multicentric
Multi-country interventional trials
Observational research
Real World Evidence
High-frequency scientific data collection initiatives
Built for Flexibility. Designed for Impact.
ECS™ combines three seamlessly integrated modules that empower participants, streamline data collection, and support AI-enhanced research coordination. Whether your study is decentralized, hybrid, or site-based, ECS™ adapts to fit your protocol and goals.

Lucah™ app
ECS™ maintains continuous participant interaction through digital reminders, mobile notifications, and live support—reducing dropout rates and improving data reliability across long-term studies.

On-Boarding
Managing people data means making them fully comfortable. The virtual home-based functionalities for D-T-P engagement process can allow Lucah™ App to manage the onboarding and eConsent phase remotely.

Study Journey
Creating a comfortable Journey when the Study is oftentimes a burden in our fast-paced society and busy lifestyle. The multichannel collection of Participant’s health data at the touch of a button: eQuestionnaires, audio/image/video capture and wearable device integration.

Study agenda planning
What if the Participant misses the IP or an assigned Study task? Forgetfulness is a Human feature, not a bug; so here is how Lucah™ can help. Schedule Study Events and Tasks and set reminders/notifications to ensure the Participant’s compliance and retention are maintained throughout the Study Journey.

Data Capture Module
Looking to engage with and manage remotely Participants through Digital Health while having access to their health data in real-time?
Looking to manage eCRF, eCOA/TeleCOA, eClinRo, eObsRo, ePerfO, ePRO, Health Surveys, eDiary, PRO/ePRO, PROM/ePROM?
The Data Capture Module can handle and roll out in an innovative and certified way all these tools with a superior user experience.

Telehealth
Integrating telehealth with other virtual health solutions and in-person/hybrid/virtual care models, to improve patient/participant experience, convenience, outcomes, and affordability.

eCRF
Flexibility and rapid set-up time of eCRFs that facilitate the collection of accurate observations and reliable findings, and support transparency by providing real-time access to data.

Remote management
The remote management better engages patients/participants in experiencing the study journey, and gives the doctor a clearer window into their progress.

Interactive supply chain tool
A comprehensive flow of D-T-P (Direct to Participant) and D-F-P (Direct from Participant) processes for IP delivery and adherence eTracking, thanks to the direct connection with certified courier for sample delivery & recollection to and from home.

Virtual Intelligent Study Assistant Modules (ViSA)
ECS™ maintains continuous participant interaction through digital reminders, mobile notifications, and live support—reducing dropout rates and improving data reliability across long-term studies.

Workflow Automation
From visit scheduling and automated reminders to data validation and quality checks, ECS™ reduces manual tasks across the study lifecycle. Your team can focus on science, not spreadsheets.

Seamless User Experience
Participants use the Lucah™ mobile app to report data, receive reminders, and attend tele-visits—all from their own device. Researchers and sponsors benefit from tailored dashboards and role-based interfaces, ensuring clarity, speed, and usability at every level.

Purpose-Built Tools for Every Stakeholder
ECS™ is not one-size-fits-all. Investigators, monitors, and sponsors each get customized access to data and study tools, while participants engage through a secure, intuitive digital environment—boosting retention and protocol adherence.
AI-Powered Virtual Study Assistant
24/7 Support
ViSA provides continuous assistance to both participants and researchers, ensuring questions are answered at any time.
Automated Scheduling
Coordinates appointments, sends reminders, and manages follow-ups to keep studies on track.
Intelligent Chatbot
Answers protocol questions, delivers health surveys, and provides personalized guidance throughout the study.
Reduced Admin Load
Handles repetitive tasks automatically, allowing research staff to focus on higher-value activities.
Transform Your Next Trial with ECS™ – Connect with us
Participant-facing mobile application for ePRO, eDiary, and telehealth visits
Get in Touch
Contact our team to discuss your specific needs
Data Capture & ViSA
Experience the platform in action
Launch Your Study
Transform your research with next-gen technology

What is ECSTM
Why ECSTM
Solutions
Therapeutic areas
Case studies
Compliance
Copyright @2025 Evidilya srl. All rights reserved.
Privacy Policy
Cookie Policy

What is ECSTM
Why ECS™
Solutions
Therapeutic areas
Case studies
Compliance

One Connected Ecosystem to Power Every Phase of Healthcare Research
Evidence Capture System ™ [ECS™] is a next-generation virtual research ecosystem designed to streamline research from start to finish. It blends fully remote study capabilities with phygital (physical + digital) flexibility, creating a seamless environment where researchers, participants, and sponsors collaborate in real time.
Faster Science, Stronger Evidence
Through end-to-end digitalization of all study touchpoints — from direct-to-participant onboarding and eConsent to real-time data acquisition and centralized monitoring — ECS™ optimizes study execution while maintaining strict adherence to GXP, data integrity principles, and international regulatory frameworks.
Architected for full scalability, ECS™ supports a wide spectrum of protocol designs:
Multicentric
Multi-country interventional trials
Observational research
Real World Evidence
High-frequency scientific data collection initiatives
Built for Flexibility. Designed for Impact.
ECS™ combines three seamlessly integrated modules that empower participants, streamline data collection, and support AI-enhanced research coordination. Whether your study is decentralized, hybrid, or site-based, ECS™ adapts to fit your protocol and goals.

Lucah™ app
ECS™ maintains continuous participant interaction through digital reminders, mobile notifications, and live support—reducing dropout rates and improving data reliability across long-term studies.

On-Boarding
Managing people data means making them fully comfortable. The virtual home-based functionalities for D-T-P engagement process can allow Lucah™ App to manage the onboarding and eConsent phase remotely.

Study Journey
Creating a comfortable Journey when the Study is oftentimes a burden in our fast-paced society and busy lifestyle. The multichannel collection of Participant’s health data at the touch of a button: eQuestionnaires, audio/image/video capture and wearable device integration.

Study agenda planning
What if the Participant misses the IP or an assigned Study task? Forgetfulness is a Human feature, not a bug; so here is how Lucah™ can help. Schedule Study Events and Tasks and set reminders/notifications to ensure the Participant’s compliance and retention are maintained throughout the Study Journey.
Data Capture Module
Looking to engage with and manage remotely Participants through Digital Health while having access to their health data in real-time?
Looking to manage eCRF, eCOA/TeleCOA, eClinRo, eObsRo, ePerfO, ePRO, Health Surveys, eDiary, PRO/ePRO, PROM/ePROM?
The Data Capture Module can handle and roll out in an innovative and certified way all these tools with a superior user experience.

Telehealth
Integrating telehealth with other virtual health solutions and in-person/hybrid/virtual care models, to improve patient/participant experience, convenience, outcomes, and affordability.

eCRF
Flexibility and rapid set-up time of eCRFs that facilitate the collection of accurate observations and reliable findings, and support transparency by providing real-time access to data.

Remote management
The remote management better engages patients/participants in experiencing the study journey, and gives the doctor a clearer window into their progress.

Interactive supply chain tool
A comprehensive flow of D-T-P (Direct to Participant) and D-F-P (Direct from Participant) processes for IP delivery and adherence eTracking, thanks to the direct connection with certified courier for sample delivery & recollection to and from home.


Virtual Intelligent Study Assistant Modules (ViSA)
ECS™ maintains continuous participant interaction through digital reminders, mobile notifications, and live support—reducing dropout rates and improving data reliability across long-term studies.

Workflow Automation
From visit scheduling and automated reminders to data validation and quality checks, ECS™ reduces manual tasks across the study lifecycle. Your team can focus on science, not spreadsheets.

Seamless User Experience
Participants use the Lucah™ mobile app to report data, receive reminders, and attend tele-visits—all from their own device. Researchers and sponsors benefit from tailored dashboards and role-based interfaces, ensuring clarity, speed, and usability at every level.

Purpose-Built Tools for Every Stakeholder
ECS™ is not one-size-fits-all. Investigators, monitors, and sponsors each get customized access to data and study tools, while participants engage through a secure, intuitive digital environment—boosting retention and protocol adherence.
AI-Powered Virtual Study Assistant
24/7 Support
ViSA provides continuous assistance to both participants and researchers, ensuring questions are answered at any time.
Automated Scheduling
Coordinates appointments, sends reminders, and manages follow-ups to keep studies on track.
Intelligent Chatbot
Answers protocol questions, delivers health surveys, and provides personalized guidance throughout the study.
Reduced Admin Load
Handles repetitive tasks automatically, allowing research staff to focus on higher-value activities.
Transform Your Next Trial with ECS™ – Connect with us
Participant-facing mobile application for ePRO, eDiary, and telehealth visits
Get in Touch
Contact our team to discuss your specific needs
Data Capture & ViSA
Experience the platform in action
Launch Your Study
Transform your research with next-gen technology

What is ECSTM
Why ECSTM
Solutions
Therapeutic areas
Case studies
Compliance
Copyright @2025 Evidilya srl. All rights reserved.
Privacy Policy
Cookie Policy