Benefits
Specifications
How-to
Contact Us
Learn More
Ready for Next-Gen, High-Impact Evidence-Based healthcare Research?
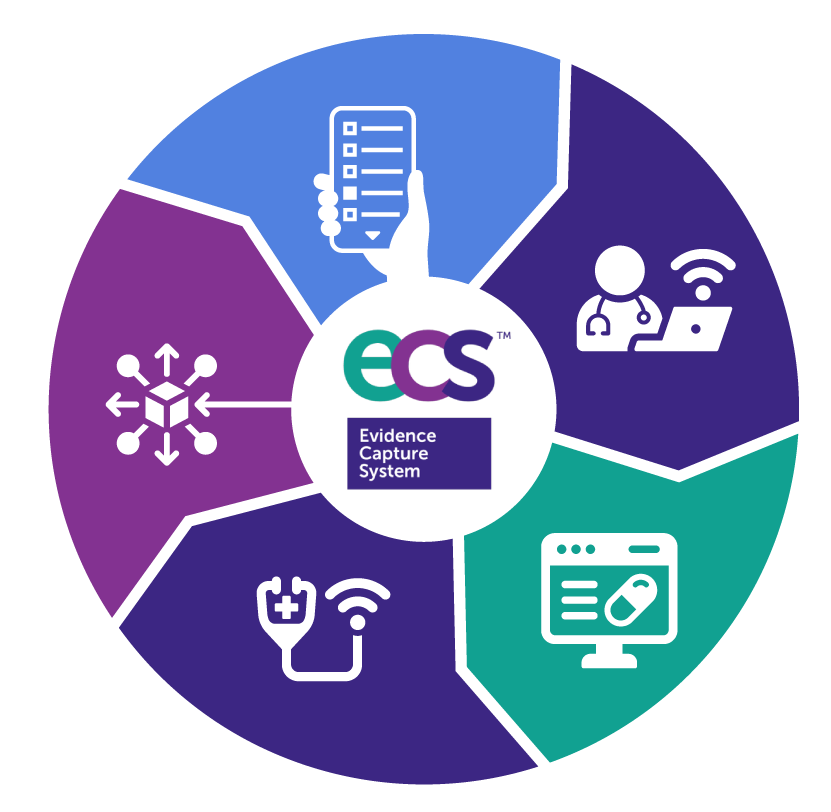
Evidence Capture System™ [ECS™] is a next-generation virtual research ecosystem designed to streamline research from start to finish. It blends fully remote study capabilities with phygital (physical + digital) flexibility, creating a seamless environment where researchers, participants, and sponsors collaborate in real time.
Powered by Evidilya – People and Research First™
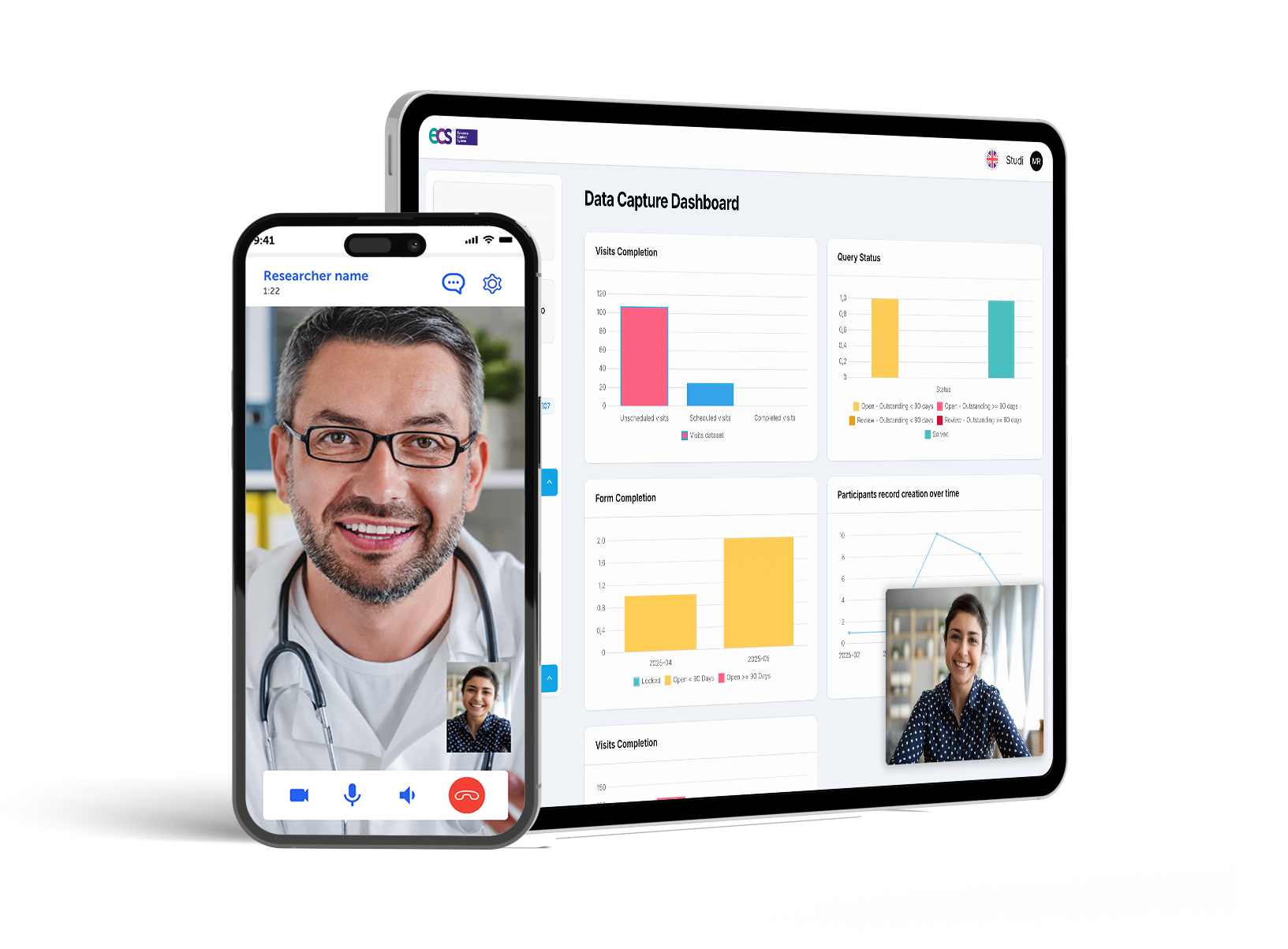
One Connected Ecosystem to Power Every Phase of Healthcare Research
ECS™ digital platform supports every study model—virtual, centralized, or hybrid—and empowers researchers in hospitals, community care settings, and decentralized networks. It delivers a seamless, engaging digital-first experience for participants.
All-in-One Study Control Platform
An innovative Ecosystem for designing and deploying your research projects. ECS™ is built from interoperable modules that activate as needed — whether you run a simple EDC or a complex, multinational DCT. Scales to your protocol and integrates smoothly with your existing workflows and systems.
LucaH App™
Participant-facing mobile application that simplifies clinical trial engagement through integrated ePRO, eDiary, and telehealth functionalities, enhancing compliance and streamlines remote participation.
Smart Supply Chain Module
Investigational Product logistics, randomization, and Direct-to-Patient shipments with full tracking and accountability.
HRDD module
EDC + EHR Integration. Seamlessly gathers health records and structured eCRF data from multiple sources — including EHR, labs, wearables — for automated ingestion and source data verification.
ViSA
AI-driven support to monitor, alert, and guide users through study procedures, aligned with risk-based management plans.Provides real-time access to study progress, alerts, compliance flags, and predictive analytics for early signal detection.
Data capture Module
Full-featured eCRF/eCTMS and eTMF functionalities with intuitive form builders, audit trails, automated queries, and role-based workflows. Includes eCOA tools for PROs, clinician assessments, and psychometric scales, with native support for validated instruments. Enables centralized, hybrid and decentralized capture via mobile, web, or remote interviews, with TeleHealth and scheduling integrated.
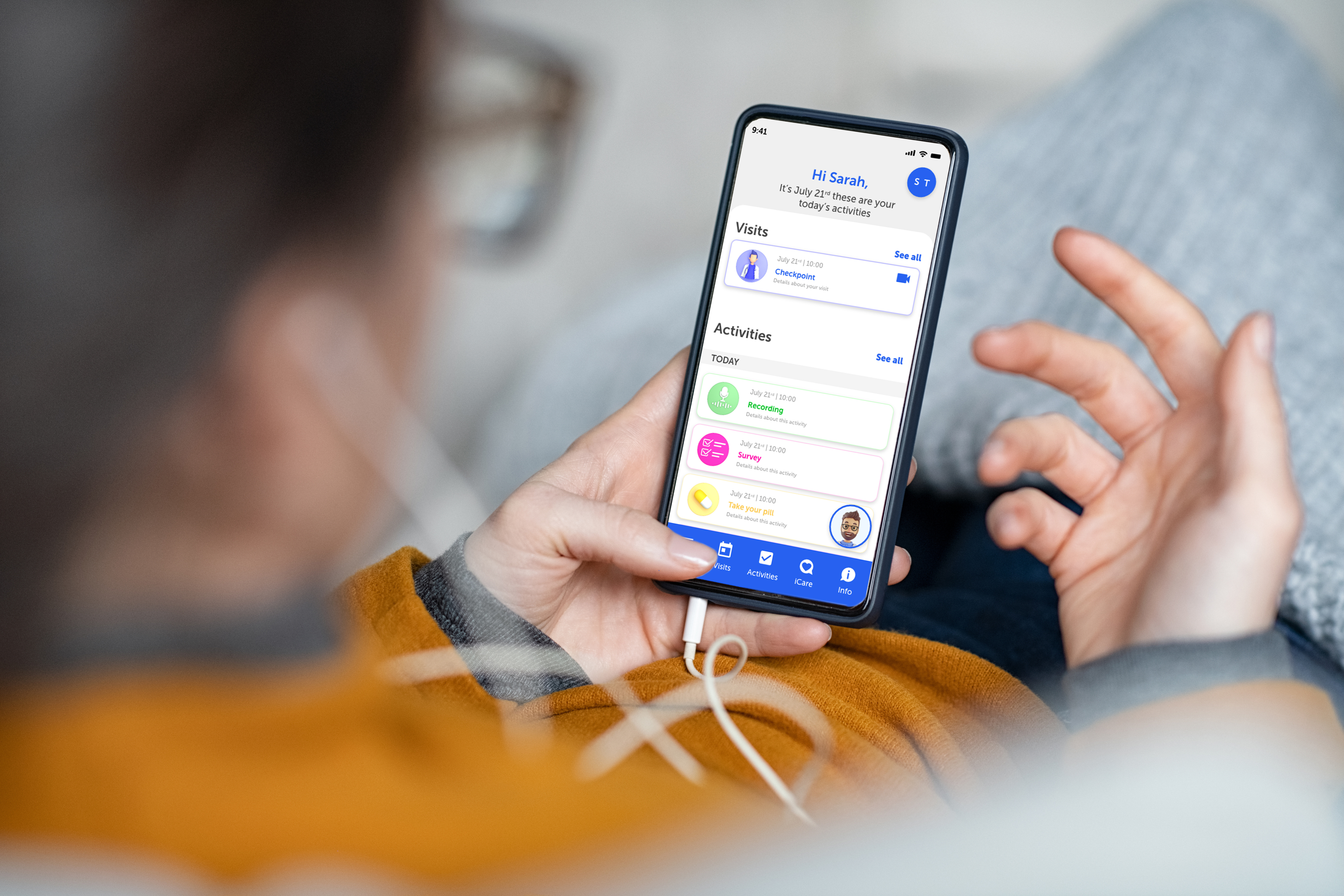
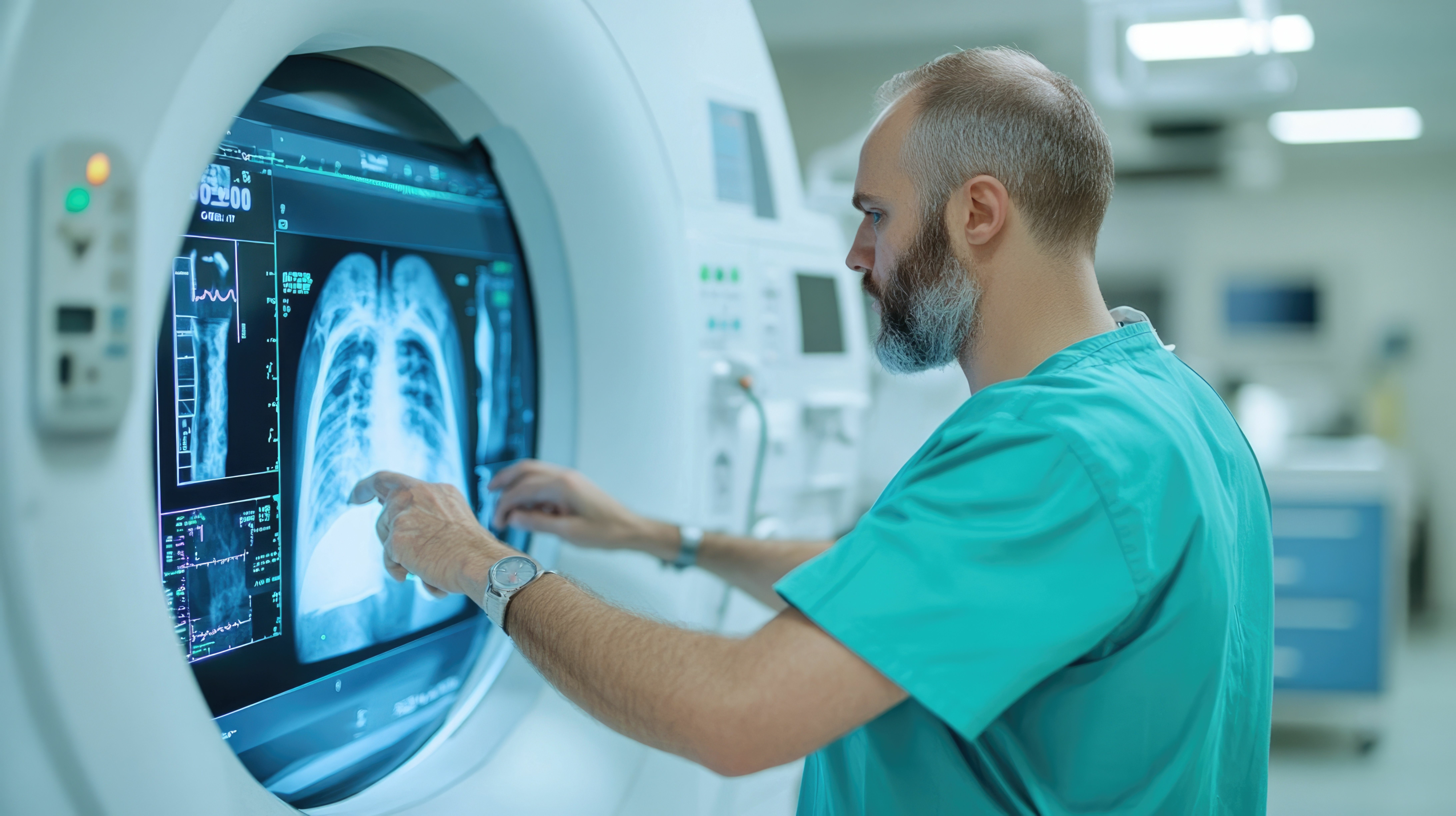

Research, Together — Wherever You Are
Decentralized & Hybrid Research Support
Run studies anytime, anywhere. ECS™ supports traditional, fully virtual (site-less), and hybrid (phygital) study designs with equal ease. Whether patients are on-site or at home, the platform ensures all processes are integrated and consistent.
Participant Journey Management
Engage participants every step of the way. Our ecosystem guides participants from eRecruitment and eConsent through daily study tasks and ePRO surveys. The Lucah™ mobile app provides a user-friendly interface for diary entries, telehealth visits, and reminders.

Real-Time Monitoring & Analytics
Immediate oversight for researchers. Investigators access incoming data instantly through ECS™'s web portal. The Data Capture Module offers real-time dashboards, centralized remote monitoring tools, and alerts for out-of-range events and data.
What Researchers Are Saying
85%
Time Saved
On administrative tasks compared to traditional research methods
3x
Faster Data Collection
With real-time digital tools vs. conventional approaches
98%
User Satisfaction
Among scientific researchers using the ECS™ platform
+25%
Participant Adherence Rate
Maintained through digital reminders and personalized support tools
ECS™ benefit in Key therapeutic Areas
Oncology
ECS™ empowers participants in oncology trials with mobile-first tools to manage symptoms and treatment routines, improving adherence throughout complex protocols. Researchers benefit from seamless integration of imaging, biomarkers, and wearable data within a single dashboard, along with the option to deploy validated QoL and psychometric questionnaires. This approach streamlines multicentric studies, accelerates patient recruitment, and reduces dropout rates.
Cardiology
Cardiology trials using ECS™ offerparticipants wearable-integrated monitoring of vitalsigns with automated alerts that enhance safety and engagement. Investigators access real-time ECGs, labs, and adherence data in a unified platform, with support for validated cardiovascular risk and lifestyle questionnaires. ECS™ accelerates site activation, strengthens protocol adherence, and reduces monitoring effort—resulting in faster enrollment and better study continuity.
Neurology
In neurology studies, ECS™ supports digital cognitive testing, remote assessments, and ePROs via validated psychometric tools. Participants face reduced burden thanks to fewer site visits and flexible engagement channels. Researchers gain structured, longitudinal data to monitor progression over time while digital pre-screening and adaptive workflows improve operational efficiency. Together, these features support high retention and improved patient matching.
Transform Your Next Trial with ECS™ – Connect with us
Participant-facing mobile application for ePRO, eDiary, and telehealth visits
Get in Touch
Contact our team to discuss your specific needs
Data Capture & ViSA
Experience the platform in action
Launch Your Study
Transform your research with next-gen technology

What is ECSTM
Why ECSTM
Solutions
Therapeutic areas
Case studies
Compliance
Privacy Policy
Cookie Policy
Copyright @2025 Evidilya srl. All rights reserved.

Benefits
Specifications
How-to
Contact Us
Learn More
Ready for Next-Gen, High-Impact Evidence-Based healthcare Research?
Evidence Capture System™ [ECS™] is a next-generation virtual research ecosystem designed to streamline research from start to finish. It blends fully remote study capabilities with phygital (physical + digital) flexibility, creating a seamless environment where researchers, participants, and sponsors collaborate in real time.
Powered by Evidilya – People and Research First™

One Connected Ecosystem to Power Every Phase of Healthcare Research
ECS™ digital platform supports every study model—virtual, centralized, or hybrid—and empowers researchers in hospitals, community care settings, and decentralized networks. It delivers a seamless, engaging digital-first experience for participants.
All-in-One Study Control Platform
An innovative Ecosystem for designing and deploying your research projects. ECS™ is built from interoperable modules that activate as needed — whether you run a simple EDC or a complex, multinational DCT. Scales to your protocol and integrates smoothly with your existing workflows and systems.

LucaH App™
Participant-facing mobile application that simplifies clinical trial engagement through integrated ePRO, eDiary, and telehealth functionalities, enhancing compliance and streamlines remote participation.
Smart Supply Chain Module
Investigational Product logistics, randomization, and Direct-to-Patient shipments with full tracking and accountability.
HRDD module
EDC + EHR Integration. Seamlessly gathers health records and structured eCRF data from multiple sources — including EHR, labs, wearables — for automated ingestion and source data verification.
ViSA
AI-driven support to monitor, alert, and guide users through study procedures, aligned with risk-based management plans.Provides real-time access to study progress, alerts, compliance flags, and predictive analytics for early signal detection.
Data capture Module
Full-featured eCRF/eCTMS and eTMF functionalities with intuitive form builders, audit trails, automated queries, and role-based workflows. Includes eCOA tools for PROs, clinician assessments, and psychometric scales, with native support for validated instruments. Enables centralized, hybrid and decentralized capture via mobile, web, or remote interviews, with TeleHealth and scheduling integrated.



Research, Together — Wherever You Are

Decentralized & Hybrid Research Support
Run studies anytime, anywhere. ECS™ supports traditional, fully virtual (site-less), and hybrid (phygital) study designs with equal ease. Whether patients are on-site or at home, the platform ensures all processes are integrated and consistent.

Participant Journey Management
Engage participants every step of the way. Our ecosystem guides participants from eRecruitment and eConsent through daily study tasks and ePRO surveys. The Lucah™ mobile app provides a user-friendly interface for diary entries, telehealth visits, and reminders.

Real-Time Monitoring & Analytics
Immediate oversight for researchers. Investigators access incoming data instantly through ECS™'s web portal. The Data Capture Module offers real-time dashboards, centralized remote monitoring tools, and alerts for out-of-range events and data.
What Researchers Are Saying
85%
Time Saved
On administrative tasks compared to traditional research methods
3x
Faster Data Collection
With real-time digital tools vs. conventional approaches
98%
User Satisfaction
Among scientific researchers using the ECS™ platform
+25%
Participant Adherence Rate
Maintained through digital reminders and personalized support tools
ECS™ benefit in Key therapeutic Areas
Oncology
ECS™ empowers participants in oncology trials with mobile-first tools to manage symptoms and treatment routines, improving adherence throughout complex protocols. Researchers benefit from seamless integration of imaging, biomarkers, and wearable data within a single dashboard, along with the option to deploy validated QoL and psychometric questionnaires. This approach streamlines multicentric studies, accelerates patient recruitment, and reduces dropout rates.
Cardiology
Cardiology trials using ECS™ offerparticipants wearable-integrated monitoring of vitalsigns with automated alerts that enhance safety and engagement. Investigators access real-time ECGs, labs, and adherence data in a unified platform, with support for validated cardiovascular risk and lifestyle questionnaires. ECS™ accelerates site activation, strengthens protocol adherence, and reduces monitoring effort—resulting in faster enrollment and better study continuity.
Neurology
In neurology studies, ECS™ supports digital cognitive testing, remote assessments, and ePROs via validated psychometric tools. Participants face reduced burden thanks to fewer site visits and flexible engagement channels. Researchers gain structured, longitudinal data to monitor progression over time while digital pre-screening and adaptive workflows improve operational efficiency. Together, these features support high retention and improved patient matching.
Transform Your Next Trial with ECS™ – Connect with us
Participant-facing mobile application for ePRO, eDiary, and telehealth visits
Get in Touch
Contact our team to discuss your specific needs
Data Capture & ViSA
Experience the platform in action
Launch Your Study
Transform your research with next-gen technology

What is ECSTM
Why ECSTM
Solutions
Therapeutic areas
Case studies
Compliance
Copyright @2025 Evidilya srl. All rights reserved.
Privacy Policy
Cookie Policy

What is ECSTM
Why ECS™
Solutions
Therapeutic areas
Case studies
Compliance
Ready for Next-Gen, High-Impact Evidence-Based healthcare Research?
Evidence Capture System™ [ECS™] is a next-generation virtual research ecosystem designed to streamline research from start to finish. It blends fully remote study capabilities with phygital (physical + digital) flexibility, creating a seamless environment where researchers, participants, and sponsors collaborate in real time.
Powered by Evidilya – People and Research First™

One Connected Ecosystem to Power Every Phase of Healthcare Research
ECS™ digital platform supports every study model—virtual, centralized, or hybrid—and empowers researchers in hospitals, community care settings, and decentralized networks. It delivers a seamless, engaging digital-first experience for participants.
All-in-One Study Control Platform
An innovative Ecosystem for designing and deploying your research projects. ECS™ is built from interoperable modules that activate as needed — whether you run a simple EDC or a complex, multinational DCT. Scales to your protocol and integrates smoothly with your existing workflows and systems.
LucaH App™
Participant-facing mobile application that simplifies clinical trial engagement through integrated ePRO, eDiary, and telehealth functionalities, enhancing compliance and streamlines remote participation.
Smart Supply Chain Module
Investigational Product logistics, randomization, and Direct-to-Patient shipments with full tracking and accountability.
HRDD module
EDC + EHR Integration. Seamlessly gathers health records and structured eCRF data from multiple sources — including EHR, labs, wearables — for automated ingestion and source data verification.
ViSA
AI-driven support to monitor, alert, and guide users through study procedures, aligned with risk-based management plans.Provides real-time access to study progress, alerts, compliance flags, and predictive analytics for early signal detection.
Data capture Module
Full-featured eCRF/eCTMS and eTMF functionalities with intuitive form builders, audit trails, automated queries, and role-based workflows. Includes eCOA tools for PROs, clinician assessments, and psychometric scales, with native support for validated instruments. Enables centralized, hybrid and decentralized capture via mobile, web, or remote interviews, with TeleHealth and scheduling integrated.




Research, Together — Wherever You Are

Decentralized & Hybrid Research Support
Run studies anytime, anywhere. ECS™ supports traditional, fully virtual (site-less), and hybrid (phygital) study designs with equal ease. Whether patients are on-site or at home, the platform ensures all processes are integrated and consistent.

Participant Journey Management
Engage participants every step of the way. Our ecosystem guides participants from eRecruitment and eConsent through daily study tasks and ePRO surveys. The Lucah™ mobile app provides a user-friendly interface for diary entries, telehealth visits, and reminders.

Real-Time Monitoring & Analytics
Immediate oversight for researchers. Investigators access incoming data instantly through ECS™'s web portal. The Data Capture Module offers real-time dashboards, centralized remote monitoring tools, and alerts for out-of-range events and data.
What Researchers Are Saying
85%
Time Saved
On administrative tasks compared to traditional research methods
3x
Faster Data Collection
With real-time digital tools vs. conventional approaches
98%
User Satisfaction
Among scientific researchers using the ECS™ platform
+25%
Participant Adherence Rate
Maintained through digital reminders and personalized support tools
ECS™ benefit in Key therapeutic Areas
Oncology
ECS™ empowers participants in oncology trials with mobile-first tools to manage symptoms and treatment routines, improving adherence throughout complex protocols. Researchers benefit from seamless integration of imaging, biomarkers, and wearable data within a single dashboard, along with the option to deploy validated QoL and psychometric questionnaires. This approach streamlines multicentric studies, accelerates patient recruitment, and reduces dropout rates.
Cardiology
Cardiology trials using ECS™ offerparticipants wearable-integrated monitoring of vitalsigns with automated alerts that enhance safety and engagement. Investigators access real-time ECGs, labs, and adherence data in a unified platform, with support for validated cardiovascular risk and lifestyle questionnaires. ECS™ accelerates site activation, strengthens protocol adherence, and reduces monitoring effort—resulting in faster enrollment and better study continuity.
Neurology
In neurology studies, ECS™ supports digital cognitive testing, remote assessments, and ePROs via validated psychometric tools. Participants face reduced burden thanks to fewer site visits and flexible engagement channels. Researchers gain structured, longitudinal data to monitor progression over time while digital pre-screening and adaptive workflows improve operational efficiency. Together, these features support high retention and improved patient matching.
Transform Your Next Trial with ECS™ – Connect with us
Participant-facing mobile application for ePRO, eDiary, and telehealth visits
Get in Touch
Contact our team to discuss your specific needs
Data Capture & ViSA
Experience the platform in action
Launch Your Study
Transform your research with next-gen technology

What is ECSTM
Why ECSTM
Solutions
Therapeutic areas
Case studies
Compliance
Copyright @2025 Evidilya srl. All rights reserved.
Privacy Policy
Cookie Policy